Heidelberg, November 6, 2025
FDA Greenlights Pivotal Study of PRECISIS’ Minimally Invasive Brain-Stimulation Therapy for Drug-Resistant Epilepsy
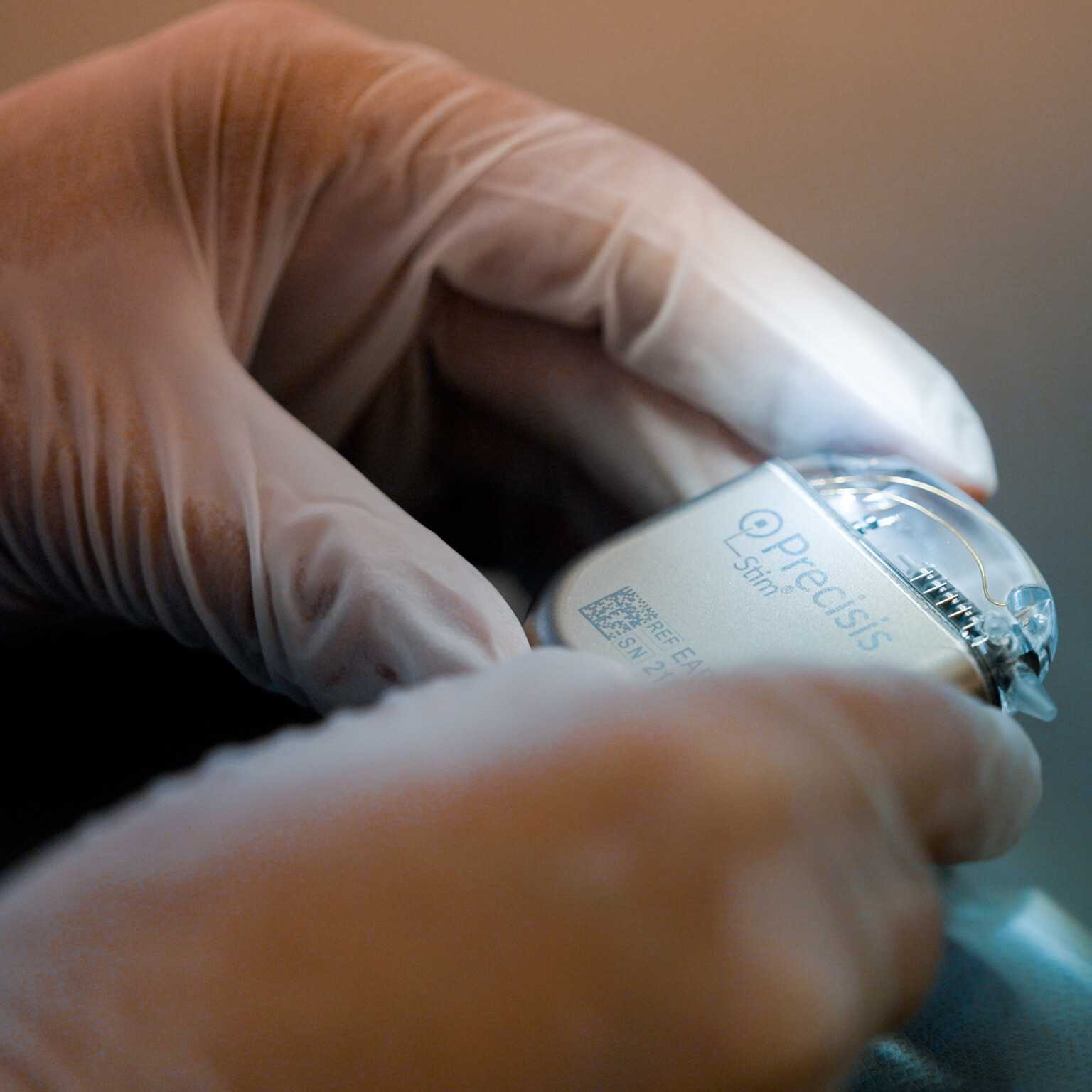
PRECISIS GmbH today announced that the U.S. Food & Drug Administration (FDA) has granted Investigational Device Exemption (IDE) approval for its pivotal EASEE4US clinical study, ma […]
Read more