An important milestone was the commencement of the pediatric study in May 2023, which will provide insights over the coming years into the extent to which the innovative EASEE® device can expand the treatment options for children and adolescents. The study is open to 12 to 17-year-olds who suffer from medication-resistant epilepsy. The study is currently underway at the following centers:
- UK Freiburg
- UK Heidelberg
- Epilepsie-Zentrum Kehl Kork
- Schön Klinik Vogtareuth
- UK Frankfurt
- Epilepsie-Zentrum Bethel, Bielefeld
- Kepler UK Linz (AU)
At the same time, the Precisis team is working diligently to introduce EASEE® into additional European markets. Based on the positive feedback from the NHS in October 2023, EASEE® is expected to be available for epilepsy treatment in the United Kingdom from Q2 2024.
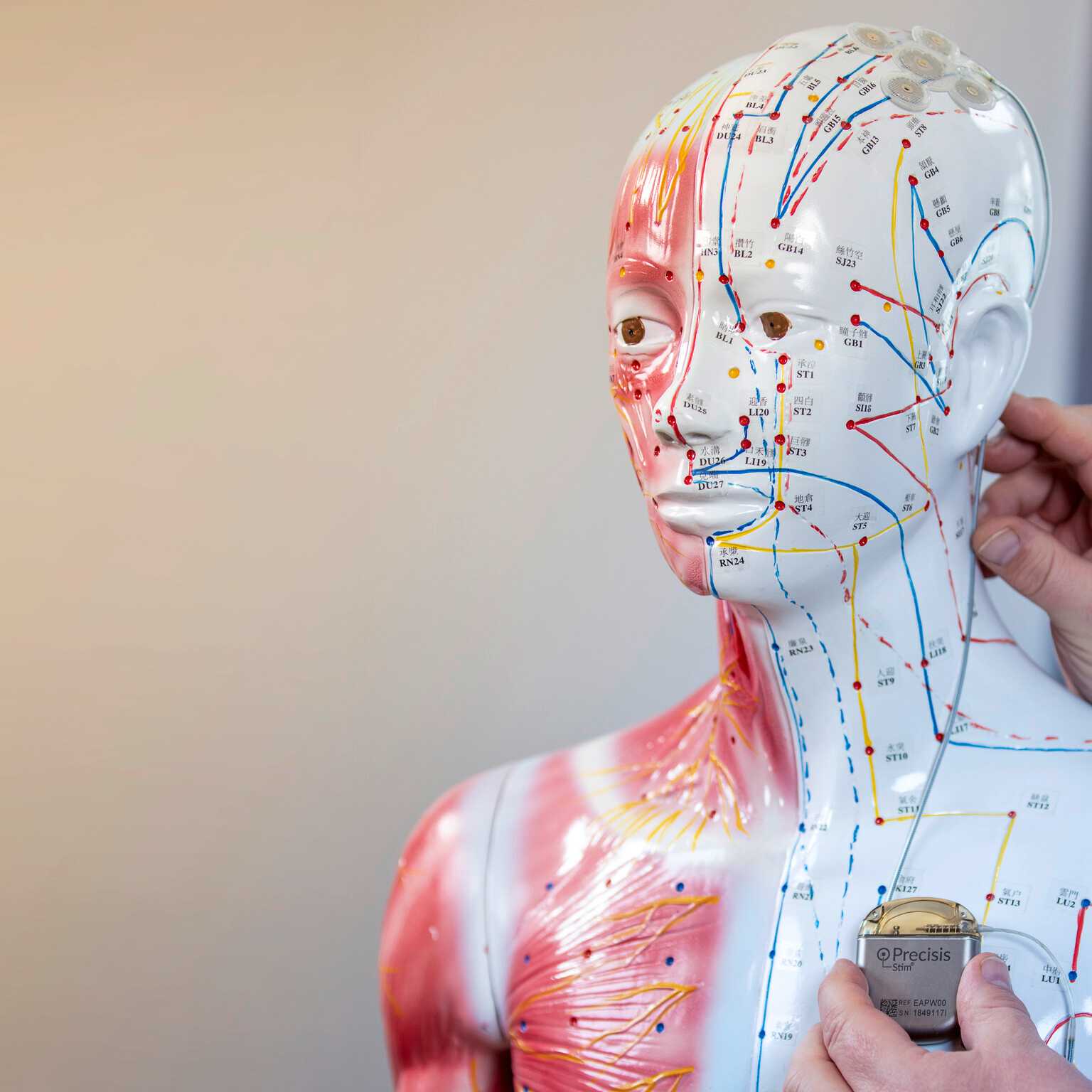
Since the EASEE® System is built on a platform technology, the device can potentially be adapted for use in other medical conditions in parallel to epilepsy.