EASEE® - The Revolutionary Implant for Drug-Resistant Focal Epilepsies

Medication
Isn't Enough
Many people with focal epilepsies rely on medication, but if seizures continue after two adequate medications, it’s known as drug-refractory epilepsy—a condition that affects millions and often leaves patients with limited options.
As the least invasive neuromodulation therapy available today, EASEE® offers a breakthrough alternative — market approved and already transforming lives across Europe. It can empower clinicians and patients alike with a safe, effective, and accessible path toward better seizure control and improved quality of life.
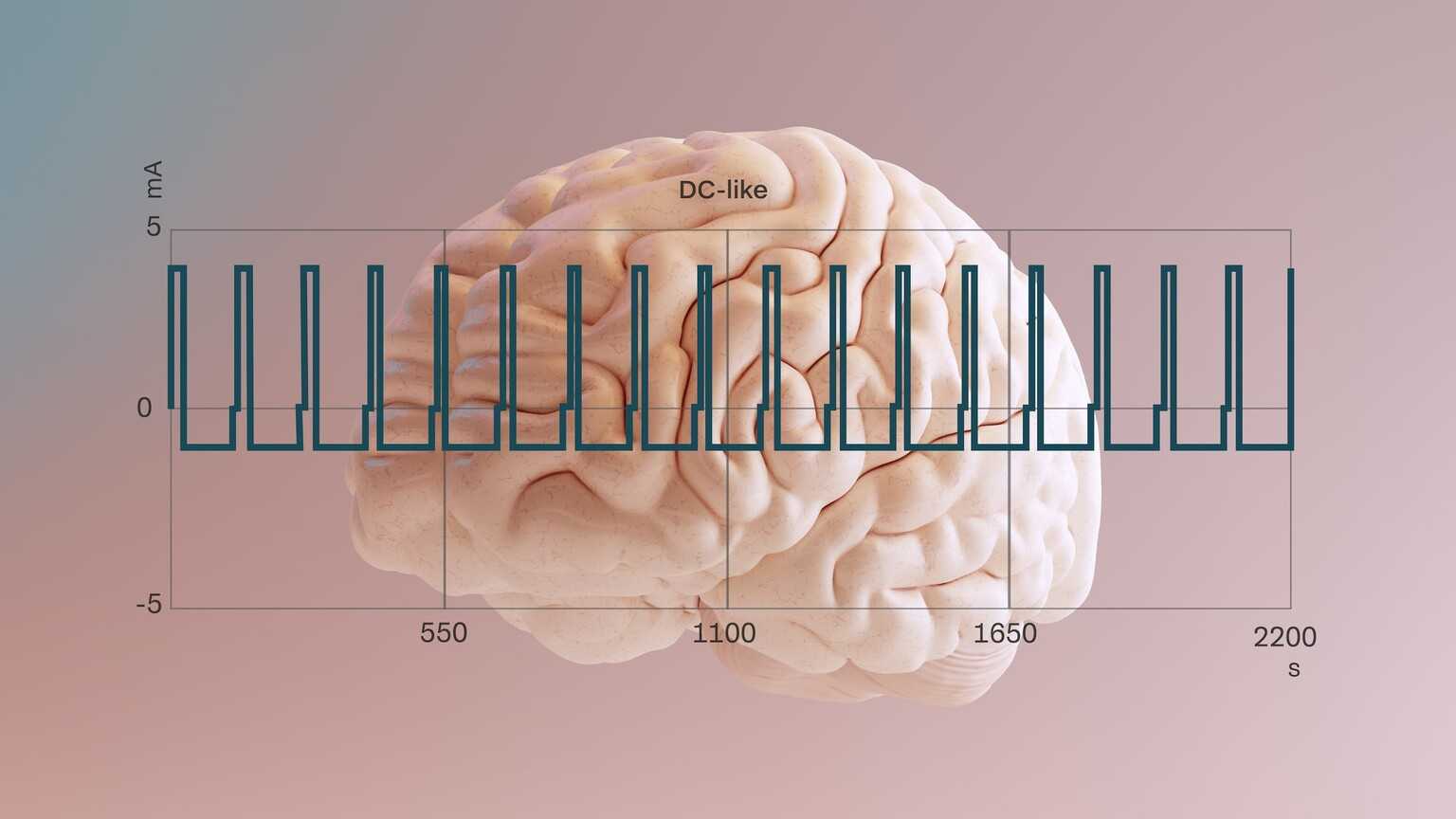
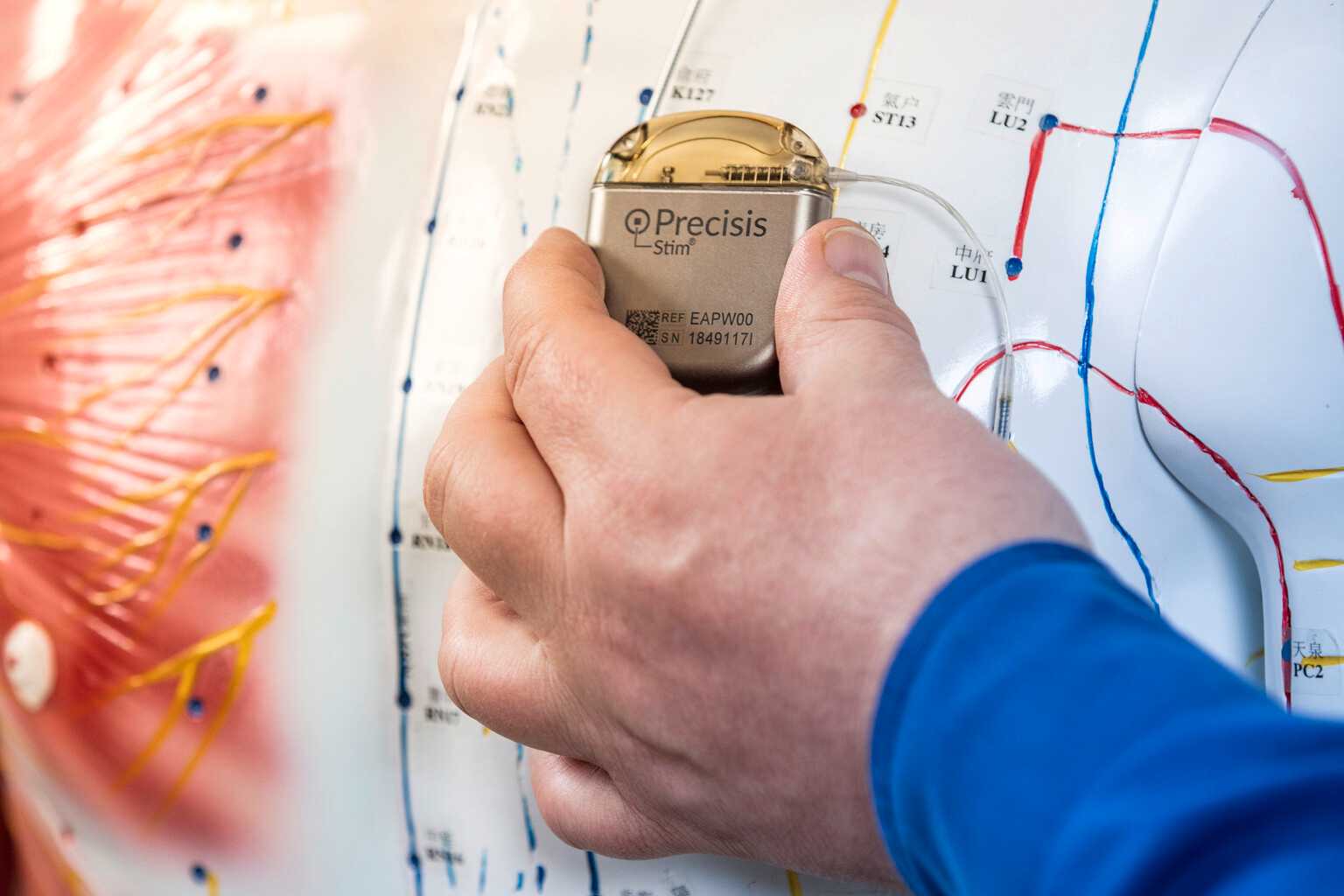
Precisis develops bioelectronic methods to direct therapeutic currents at defined target areas of the brain. AI-based learning models make it possible to activate or inhibit specific brain cells. Precisis has achieved its greatest success to date with EASEE® in the treatment of epilepsies. EASEE® is used to suppress epileptic seizures. About EASEE®.
The Precisis team is already working on the next generation of brain stimulators. The applications of targeted stimulation of cortical brain tissue are expected to be expanded to include areas such as "depression therapy" and "stroke medicine."
About the company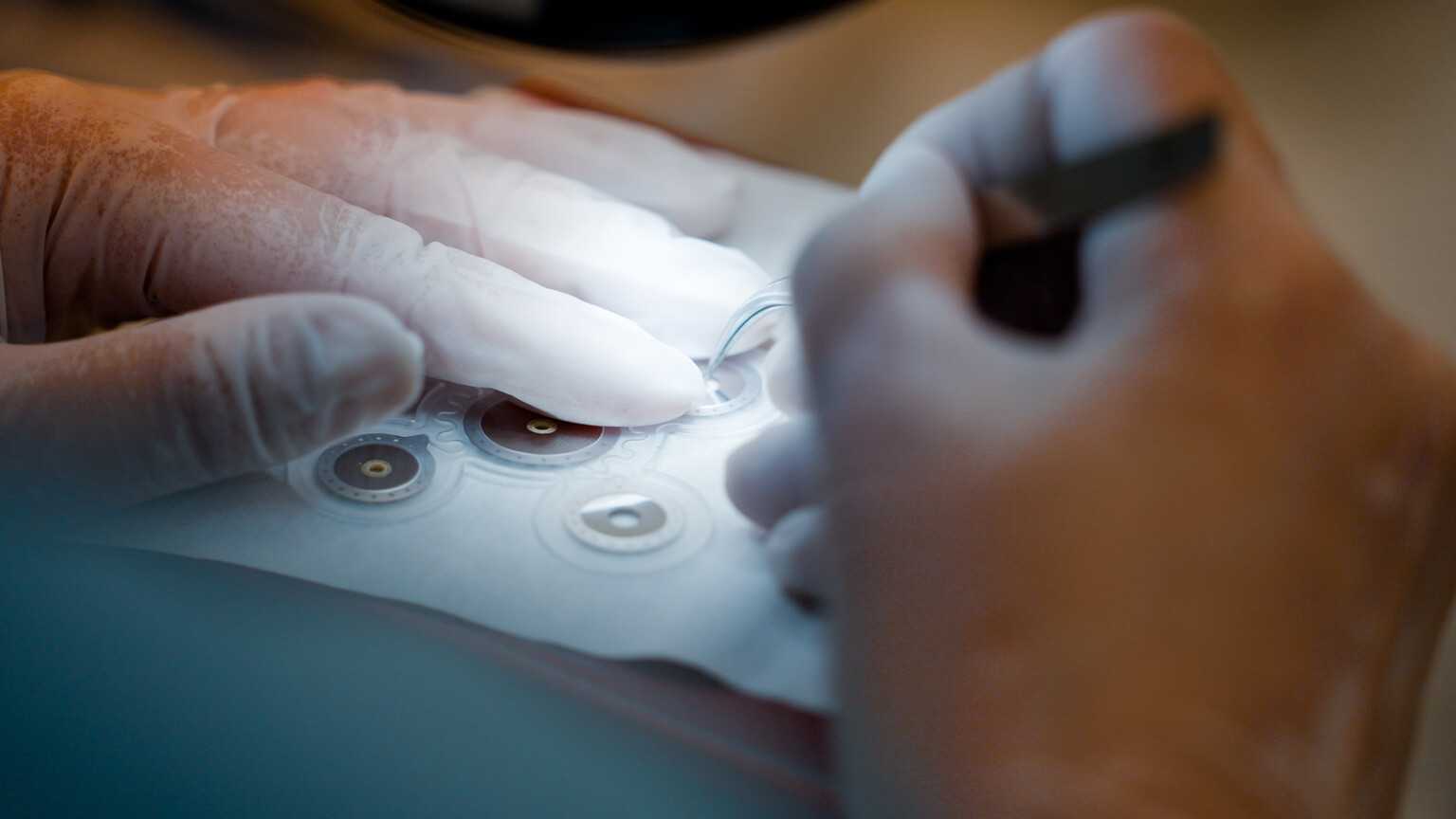
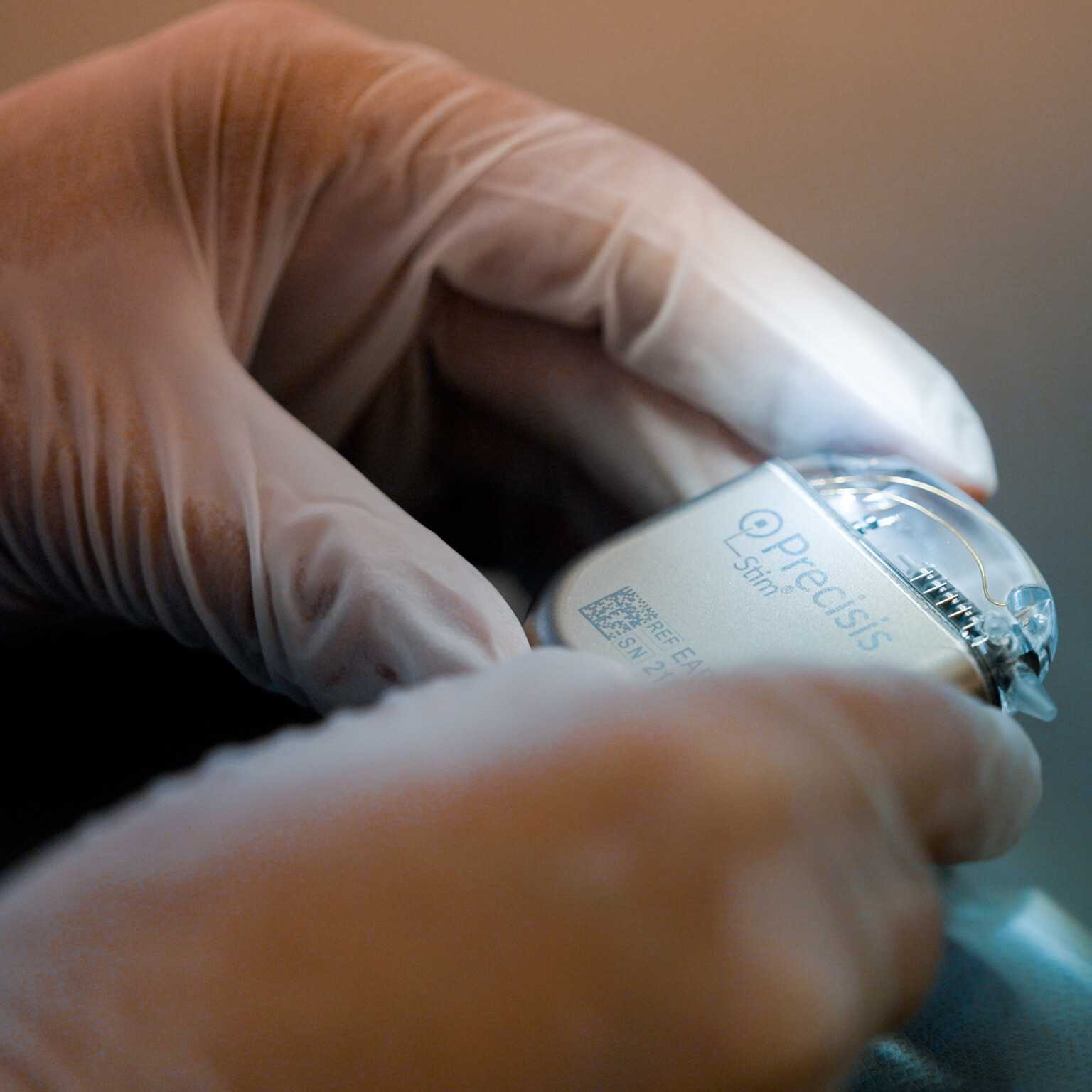
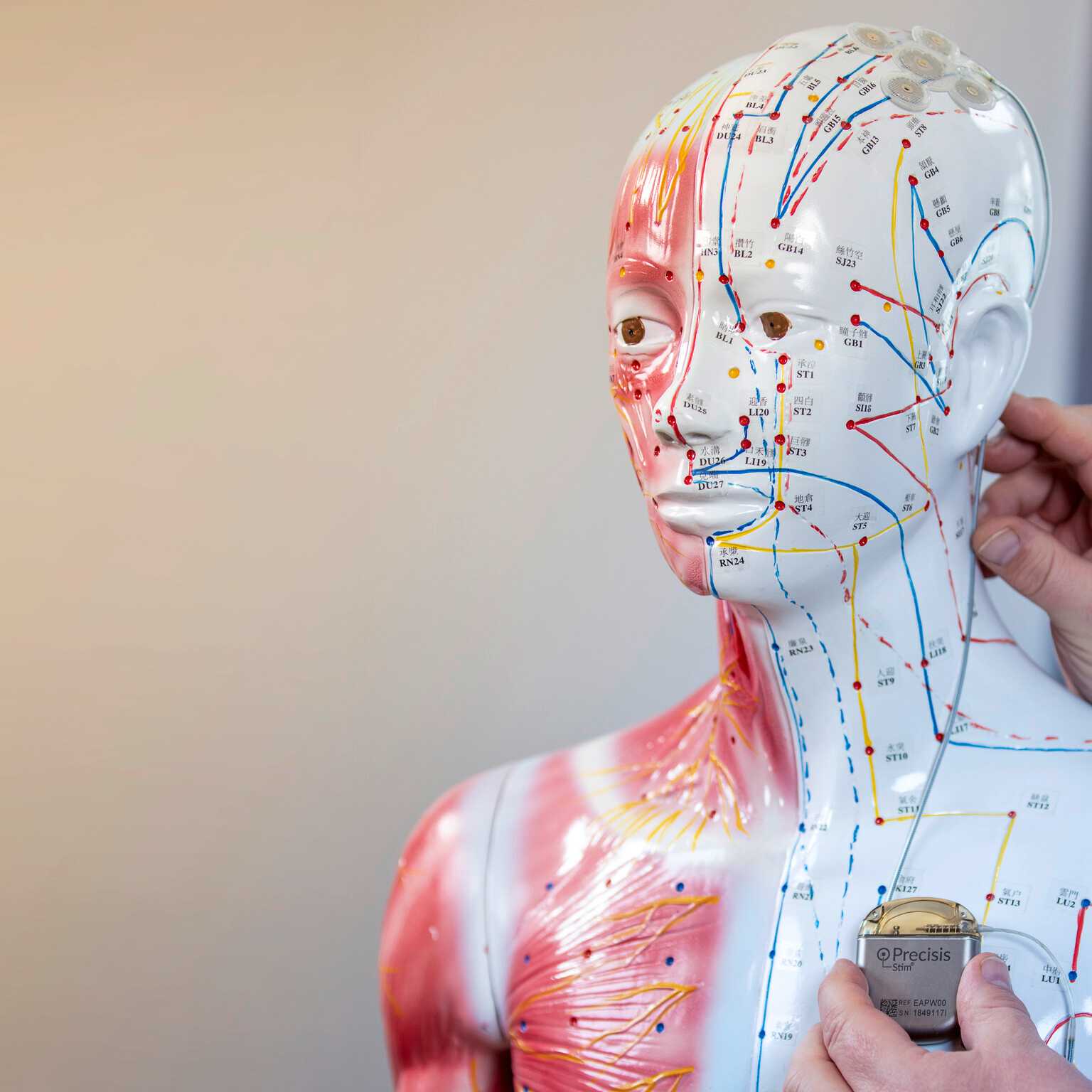
The unique achievement of the Precisis team has been to miniaturise bioelectric pacemakers for the brain and thereby make them so easily tolerable that patients will accept them as an integral part of their body. Not visible externally, the thin electrodes lend consistent support to our patient as though they were a natural means of protection against transmission malfunction in the brain.
Our core competenciesStimulation with EASEE® is a pioneering procedure that employs subcutaneous electrodes that are situated outside the cranium.

The Precisis team is made up of extraordinarily diverse personalities: they may be cautious, adventurous, scholarly, well-spoken, quiet or outspoken individuals, both world travellers and homebodies, some highly experienced and others still quite young. However, they all share one thing: each team member is the very best person for their respective position.
Become a part of this excellent team! Apply now.